 |
 | ||||||||||||
|
|||||||||||||

Why does ice float?
This was a good chance to talk about the concept of density, Archimedes running naked down the street, and helium balloons. I actually spent 2 of the weekly hours on this. I brought in a block of styrofoam, a half brick, a chunk of 4x4, and a block of ice, plus of course a big container of water. We measured volumes, something the 6th graders should be familiar with, and we measured the mass by weighing the objects on kitchen scales. Then we calculated density by dividing the two. It is important to do this in metric units (centimeters and grams), because 1 cm**3 (cubic cm) weighs exactly 1g, and this is of course no accident. Sure enough, according to the measurements, ice and wood floated, and we predicted just how deep the foam block would sink. The second week we explored the curious fact that the density of ice is less than that of water (see the links below), and talked a bit more about buoyancy of balloons and such, since it was clear the first week that they had trouble making the leap from wood floating on water to helium balloons rising in air.
Links:
|
|||||||||||||
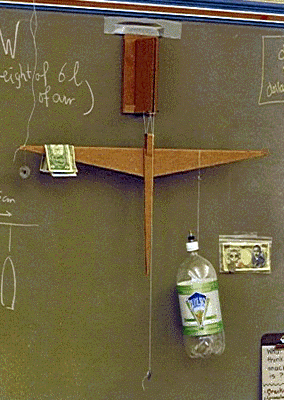
How much does air weigh?
The previous topic of density, floating and all that naturally led me to helium balloons, and why they rise. I think the kids had trouble thinking of air as a fluid, much like water, so I decided we should weigh the air in order to make this a little clearer.
How do you weigh air using only simple materials? Here is how we did it:
(4) Now we take off the bottle and put some more air in it. Tighten the cap and start pumping. How can you tell how much air you have added to the bottle? By measuring the pressure! Use a tire gauge to check every now and then, or if you are lucky, you have a bicycle pump with a pressure gauge attached. Normal air pressure is 15 psi, so if the gauge reads 15 psi, that means you have added 2 liters of extra air to your 2-liter bottle. I pumped mine up to 45 psi [these bottles can survive pressures of much more than 100 psi, so this is quite safe]. At 45 psi, you have therefore added 6 liters of air to the bottle. (5) Hang the bottle back exactly on the mark where it was before. It is clearly heavier now! Re-balance the beam using standard weights. The standard weights I know of are:
(as you can maybe see in the photograph, this is US currency, the ugliest and worst-designed currency in the Western world). On the photo we have 4 dollar bills draped over the left side to balance the extra 6 liters of air. How do we now calculate the weight of 1 liter of air?
(6)
We make use of the fact that a beam balance is a multiplication
machine, that is:
where of course W_6liter is the weight of 6 liters of classroom air. The weight of one liter of air is then 4×24.5/14.2/6 = 1.2 grams. Bingo!
When I did this the next year in mr. Waechter's class, my big footpump was
broken (too much abuse from launching water rockets), and all I had was
my small hand pump. To save time, I did it with a 1-liter bottle, pumped
to only 15 psi. On the left I had a single dollar at 28.5 cm, and on the
right I had 1 liter of extra air out at 24.5 cm. Therefore
Links:
|
|||||||||||||

Why do dogs have fur?
This is about warm-bloodedness (endothermy), cold-bloodedness (exothermy) and gigantothermy (keeping warm because of huge size). We went back to the dinosaur age and discussed how being warm-blooded could be a great advantage then: you could operate in environments where other small, coldblooded, animals could not: in cold climates, and at night. An ecological niche ready to be taken over by mammals. Being warmblooded costs lots of energy, to keep the internal heater going, and to conserve all that energy, fur is needed as an efficient insulator. We sidetracked a bit about methods for temperature regulation: sweating, panting, radiators (as in elephants ears and cows horns) and the like. I explained the third way of keeping warm: being really big. Currently, there is a lively debate among paleontologists about whether the giant dinosaurs were coldblooded or not. Hints come from fossils, and from living giant reptiles, such as giant sea turtles, and big crocodiles and alligators. Most of the links below touch on this ongoing debate.
Links:
|
|||||||||||||

If you sent a balloon to the bottom of
the ocean, how come it will shrink?
To six-graders, the answer to this was not intuitively obvious. I usually ask for guesses before I say anything on the subject myself, and I got 3 possible explanations: 1) things shrink when they get wet, 2) there is less air at the bottom of the ocean, and 3) it has to do with pressure. Hmm... Now that I had a pressurizable bottle from one of the previous experiments, this was easy to show. Here again are all the ingredients:
Look at the previous experiment for instructions on how to put together the bottlecap and valve. Take a length of string and tie one end to the cap and valve, and the other around the neck of the bottle.
This is where the straw comes in. Thread some string through the straw, and put a knot on both sides so it doesn't fall out. Now slide the straw into the bottle alongside the balloon, before you start blowing it up. Let the straw stick out at the top a little bit. Now when you blow up the balloon, air can leave the bottle through the straw. The balloon will try to flatten the straw against the inside of the bottle, but the string will prevent that for a while. When you can no longer get any more air in, tie a knot in the balloon, and stuff the end, as well as the end of the straw, into the bottle, and put on the cap. If all goes well, the balloon now fills about half of the 2-liter bottle Start pumping! As the pressure rises, the balloon will shrink, until it is so small it can bounce around inside the bottle. The higher the pressure, the smaller the balloon. If you hold the bottle right side up, and slowly let some of the air out, the balloon will expand again, and eventually will once again take up 1/2 of the bottle.
Can you ever get this balloon back out of the bottle again? We pumped the bottle back up to shrink the balloon, and then turned it over so the balloon fell to the end where the cap was. Now when you let out some air, the balloon will get sucked into the neck of the bottle, and block the valve. No more air can come out, and there is still plenty of pressure in the bottle. If you now unscrew the cap, the air will come out with a tremendous bang. In fact, if the cap were not tied to the bottle, it could fly off and do some damage. Of course, in the process, not much is left of the balloon, but the kids love the explosion. If you choose to do this, do use caution. Keep a firm grip on both the bottle and the cap, and have no one stand too close. One thing leads to another, and the following additional questions came up: Could you do this in a pool? The pressure at the bottom of the deep end is about 1/3 atm, a lot less than you can achieve in the bottle (3-4 atm). However, I bet that if you tie a sting loosely around a balloon, and take it down to the bottom of the pool, the balloon will shrink enough for the string to fall off. Why don't people shrink when they dive to the bottom of the pool? Solids and liquids are pretty much incompressible, so the body stays the same size. However, in places where there is air, as your ears and lungs, things do get squeezed. I discussed the eardrum and the middle ear in terms of what we had just seen; The eardrum is in fact very much like a piece of the balloon. You can not feel the effect of the pressure on your hands, but you do feel it on your eardrums, because the air in the middle ear gets compressed. This led to popping ears in airplanes, and the function of the Eustachian tubes. All in all an hour with very good detours.
Links about air pressure
|
|||||||||||||
| |||||||||||||

 The bottle cap with the tire valve mounted
in it
The bottle cap with the tire valve mounted
in it
 The balloon takes up more than half of this
2-liter bottle. Note the straw alongside.
The balloon takes up more than half of this
2-liter bottle. Note the straw alongside.
 By pumping up the bottle, the balloon inside has
now shrunk so much that it fell to the bottom
By pumping up the bottle, the balloon inside has
now shrunk so much that it fell to the bottom